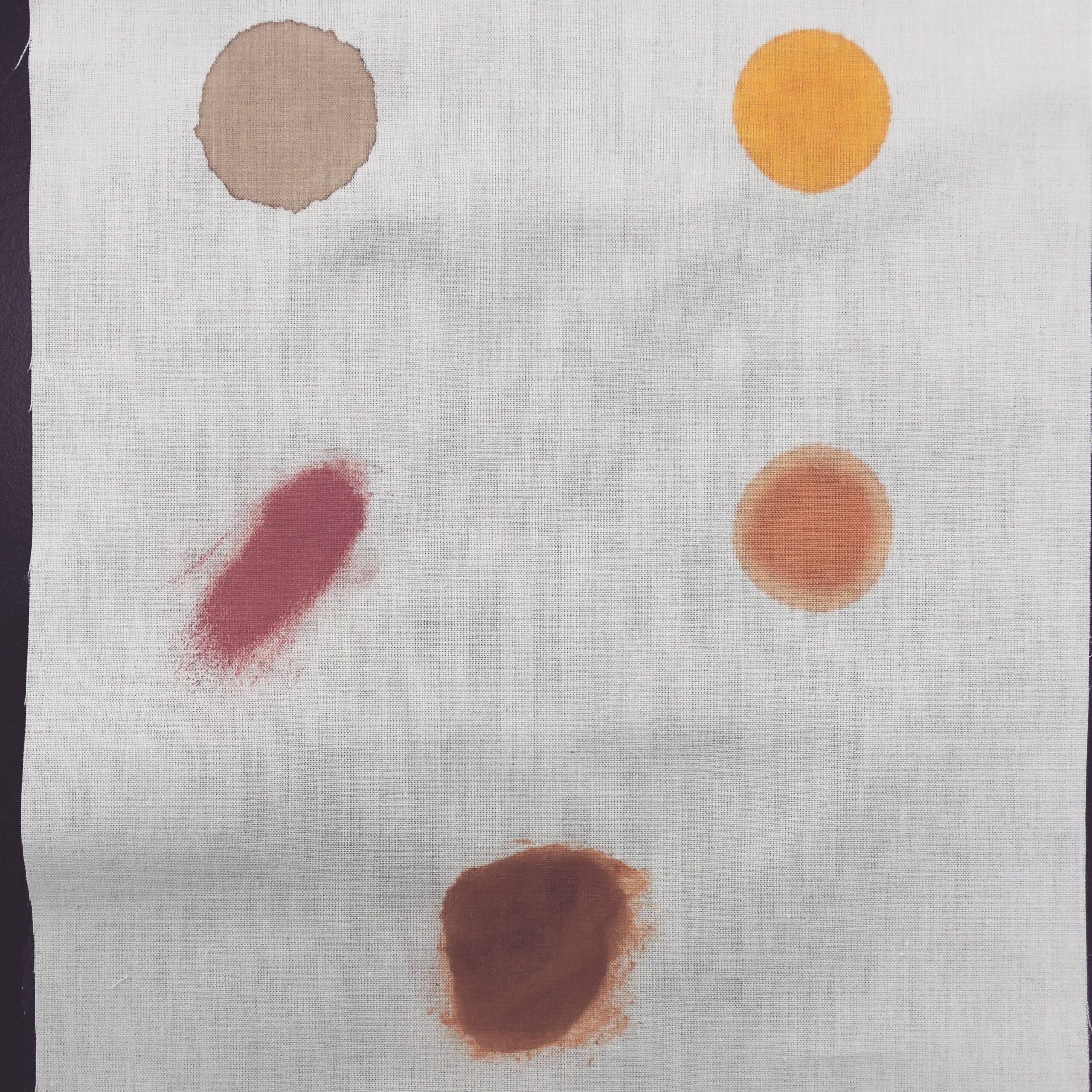
The process of laundry starts with stains. They each make their own demands and raise questions about how to get the needed result. So how does a stain impact the best use of chemicals, water and time? What should we not use on certain stains?
Staining is the discoloration caused by a specific substance or material that has come into contact with a fabric. Stains can be caused by a variety of substances, such as food, beverages, ink, oil, or rust. They are often absorbed into the material and can leave a permanent mark once the body of the stain has been lifted.
The content of a stain can tell you directly how to deal with it, so in this post we’re going to get right into that.
This is one of our longer posts. There’s a progress bar around the halfway mark.
The Big Five
We’ve come up with five main ways to categorise the components of stains, to help you choose how to remove them: grease, particulate, dye, protein and metal. A stain can be made up of one or several of these, for example many kinds of make-up contain greases and leave behind particulates or even dyes.
Grease
Pretty much the number one problem in laundry is grease.
Greasy stains are made up of oils, fats or waxes, which are hydrophobic, which means they do not mix well with water. When a greasy substance is spilled or smeared onto a surface, it tends to cling to that surface and create a film, which then resists water-based cleaning solutions. Even for flat, smooth surfaces such as glass, simply wiping the surface with a wet, soapy cloth may not be enough to remove many greases. Fabrics are doubly challenging because of the structure of the fibres, limitations in the ways we can treat them and how well grease binds to the materials fabrics are made of.
When wet cleaning, emulsifiers are the surfactants of choice because they help displace greases from fabrics and keep them suspended as droplets or particles in water. Otherwise, a non-water solvent could dissolve the oils and fats, which makes dry cleaning very effective at removing them, even if we don’t agree with it as an approach. Common solvents for greasy stains include kerosene, acetone and amyl acetate. Spotting agents for grease removal generally contain a mixture of solvents and surfactants that can be rinsed away together with water.
It’s important to remove greases before other processes in the wash. Greases act as a barrier to the action of wash liquor and can react with chlorine bleach to set the grease in, turning it into something more like the greasy build up on cooking trays. Therefore it’s even more important to have a good eye for which stains contain greases and remove them as early as possible. Oily foods, kitchen grease, engineering stains, candle wax and make-up are our number one greasy culprits. We recommend emulsifiers and spotting agents to remove these.

Particulate
Particulate stains contain solid particles that are very small, typically smaller than the human eye can perceive. The smaller the particles, the more difficult the removal process tends to be. Particulates are difficult to remove because they are a bit like beach sand: they get everywhere. They can spread around and they can take awkward shapes that make the task of cleaning them like picking up a microscopic penny from a flat surface. They are also made of a wide variety of materials, so there’s no universal approach to getting rid of them that works every time.
There are three approaches to removing particulates. The first is to use a high-suspension formulation in the main wash to hopefully lift the particles off the fibres and keep them lifted. Most fully built main wash formulations are made with this in mind. At one time the industry used high-phosphate blends, which were very effective at this kind of job, but their effect on the environment led to justified restrictions in their use.
The second approach is a spotter that does the lifting and suspending as part of its action. This really depends on the exact nature of the stain, so in this case your experience at using spotters will drive the choice you make.
The final alternative is to use a chemical that reacts with the particles to make them completely dissolve away. This only really works when the particulate is some kind of mineral, like clay, concrete dust or soil, or even many kinds of powdered make-up. It’s not our first recommendation, but our rust remover can work for these just fine, provided they aren’t coated in a grease barrier.
Protein
The proteins like those in bodily fluids, sweat, blood or egg are removed well by a non-bio detergent main wash cycle. However, they can still become a problem under two conditions:
- The temperature ramps up too quickly. Temperatures only a little too far above body temperature will literally cook the protein, like frying an egg directly into the fabric. This cannot be reversed in a wash. Uncooked protein stains usually just need time to hydrate and loosen in the main wash or as part of a cool pre-wash. We don’t recommend fast temperature ramping anyway, as this can be bad for some fibres.
- The stain is bleached with a chlorine bleach before it is mostly removed. Bleached protein stains can become yellow or even brown and tend to set in when exposed to hypochlorite.
Protein stains do need to be removed effectively, even if they’re invisible, because even after a sanitiser they can become foul over time. They’re great food for microorganisms like bacteria and mould and could act as a magnet for certain other sources of discolouration.
Effective removal of protein stains, even ones cooked in by temperature, can be done by using a biological detergent blend. These contain protease enzymes that cut up the protein molecules into much smaller molecules that don’t coagulate and can be dissolved in the wash water.
Dye
Once everything else has been removed, one of the enduring problems people face is often based on a dye. Whether it’s ballpoint or curry, that last mark on an otherwise stripped piece of table linen is likely either a vegetable dye or an artificial one.
There are two fairly reliable ways to remove dyes. The first is the bleaching phase of the wash (see our post on Anatomy of a Wash Program for more information on the phases of a cycle). The idea is that once the only remaining part of the stain is the dye, the bleach can get right in there and react with the dye to make it colourless. We talk more about how bleaches work in our blog post, How Bleach Works.
The second is using a spotter. An appropriate spotter before a cycle can often at least partially remove a dye so that the cycle can be even more effective at removing whatever is left. Spotters for this tend to be good at removing greases as well, so for the more irksome food stains a spotter could be the way to go. We recommend using spotters before the cycle, so the cycle itself doesn’t get a chance to set it in.

Metal
Finally, rust and metal are a special breed of stain, formed either by friction or dissolved metals. This includes laundry encrustation due to water hardness and poor mixing of powder detergents, concrete, and iron stains that form when dissolved iron oxidises inside or on the fibre. While many metal stains are occupational such as tray rub on chef’s whites, sometimes they can form in the laundry due to rusty piping, poor water quality or just ion exchangers and filtration units that haven’t been replaced or serviced often enough. We have seen un-serviced equipment ruin a load or two with iron stains before, so do make sure you’re up to date on all your servicing.
Rust removers can vary in the amount and type of metals they can act on, but all of them are acidic or based on an acid. While we don’t usually like to talk about our products directly in our laundry science posts, we do sell two products that provide this: rust remover and eco-sour. For the most part, the product of choice for metal marks and rust is rust remover, but eco-sour can see application against calcium deposits. Encrustation of laundry, just like the scale in a kettle, is usually fixed with acidity. This can be provided by eco-sour, used as directed, though this may require a higher temperature to get the result you want.
Metal marks from friction respond well to an appropriate metal or rust remover, depending on the metal. While there’s no easy way to know whether a customer’s article is marked by steel (which can resist removal) or aluminium (which resists less), the only course of action is a rust remover, which will hopefully dissolve the metal. As far as we’re aware, the friction that causes this may well be worse for the fibre than the stain itself and it is reasonable for the customer to accept the permanence out of experience.
Any kind of rust or yellow iron mark however is crucial to remove completely. Iron on cottons accelerates the breakdown of the fibres, causing holes and tears and costing everyone money. Rust removers completely remove the iron so that this doesn’t happen. Never apply chlorine bleach to rust or iron stains, as the reaction of this bleach with the metal can leave permanent marks. This includes otherwise mild or invisible iron staining from trace iron that gets into a laundry’s water supply. Peroxides shouldn’t be used with iron stains or water that’s high in iron for a different reason: the metal in this form accelerates the breakdown of the peroxide, limiting the activity of the bleach and causing waste. We recommend removing iron stains as soon as possible, preferably before the wash.
About This Series
Our laundry science posts are here to give you the how and why of laundry processing to empower our customers and the public. If you have something you really want to know about, why not give us a call, or email us through our contact page.